Lorem ipsum dolor sit amet,consectr
At vero eos et accusamus et iusto odio dignis
At vero eos et accusamus et iusto odio dignis

At Neurosync Brain Treatment Centre, we believe that everyone deserves a life unburdened by the challenges of mental and neurological conditions. That’s why we proudly offer rTMS Therapy (Repetitive Transcranial Magnetic Stimulation)—a groundbreaking, non-invasive treatment designed to restore balance to brain function and bring hope to those struggling with conditions like depression, anxiety, PTSD, and more.
Life’s complexities can leave many feeling trapped in cycles of mental or neurological distress, but rTMS Therapy provides a path to healing by gently stimulating targeted areas of the brain to improve its functioning. With our compassionate care, state-of-the-art technology, and personalized treatment plans, we are dedicated to empowering individuals on their journey to recovery.
When you choose Neurosync, you’re choosing a team that prioritizes your well-being, understanding that every brain is as unique as the individual it belongs to.
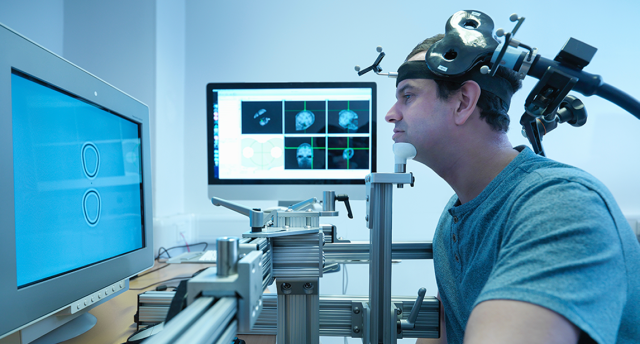
Our brains provide a complex network of neurons which communicate through electrical impulses. rTMS works by modifying electrical activity through delivered electromagnetic impulses and can influence the functioning of specified brain regions.
Repetitive Transcranial Magnetic Stimulation is a noninvasive technique that uses magnetic impulses to focus on specific areas of the brain. A coil is placed on an individual’s head, sending magnetic pulses to a targeted brain area. This type of therapy assists in regulating neuron activity, thus rewiring brain circuits.
Several conditions are treatable through rTMS, including:
Our brains are comprised of numerous interconnected areas. Each area is responsible for different functions like emotion, movement, and cognition. Regions communicate with other areas through established neural pathways. Some brain areas may show abnormal connectivity or activity patterns, causing psychiatric and neurological issues.
rTMS targets brain areas that are not correctly functioning (determined by qEEG and other diagnostic methods) to normalize activity. Using electromagnetic pulses, it modulates the neuron’s electrical impulses in the targeted area. Targeted stimulation can decrease or increase neural activity depending on the treatment’s parameters.
rTMS uses magnetic waves to gently stimulate brain activity. It does not involve injections or surgery, making it a non-invasive option compared to electroconvulsive therapy (ECT). There is no need for anesthesia or sedation, which helps minimize risks. Additionally, very few side effects have been reported, with only a small percentage of clients experiencing headaches.
In clinical settings, 75% of clients receive a response marked by decreased symptoms. Half of all clients experience remission (one to two mild symptoms remaining), and improvements are noted within the first few weeks of treatment.
Furthermore, rTMS has been noted to improve memory, attention, and executive functioning (reasoning and decision-making).
At Neurosync Brain Treatment Centre, we take a personalized and comprehensive approach to your care, beginning with a thorough initial assessment. This critical step ensures that your rTMS therapy is tailored to your unique needs and brain activity.
During the consultation, we conduct advanced diagnostic evaluations, including a qEEG (quantitative electroencephalogram) and EKG (electrocardiogram). These non-invasive tests provide detailed insights into your brainwave patterns, brain-heart coherence, and heart rate. The data is then processed through our specialized platform, comparing your brain activity against a database of nearly 30,000 recordings to identify specific areas of dysfunction.
The resulting analysis allows us to create a precise, individualized treatment plan based on your brain’s unique map. This initial consultation, lasting approximately one hour, is the foundation for effective and targeted therapy.
During your first appointment, we perform a mapping process. Clients are taken into our treatment room, seated in a reclining chair, and given earplugs to wear during the session. During a session, clients can expect:
Clients remain awake during the sessions and can resume normal activities following treatments without requiring anesthesia or medication. They stay comfortably seated in a recliner during treatments.
After three weeks, clients receive an additional ten-minute recording to examine changes in their brainwave activity. After ten days, it is objectively measured using sequential recordings. Clinical progress is measured using third-party neurocognitive assessments, instruments, and questionnaires.
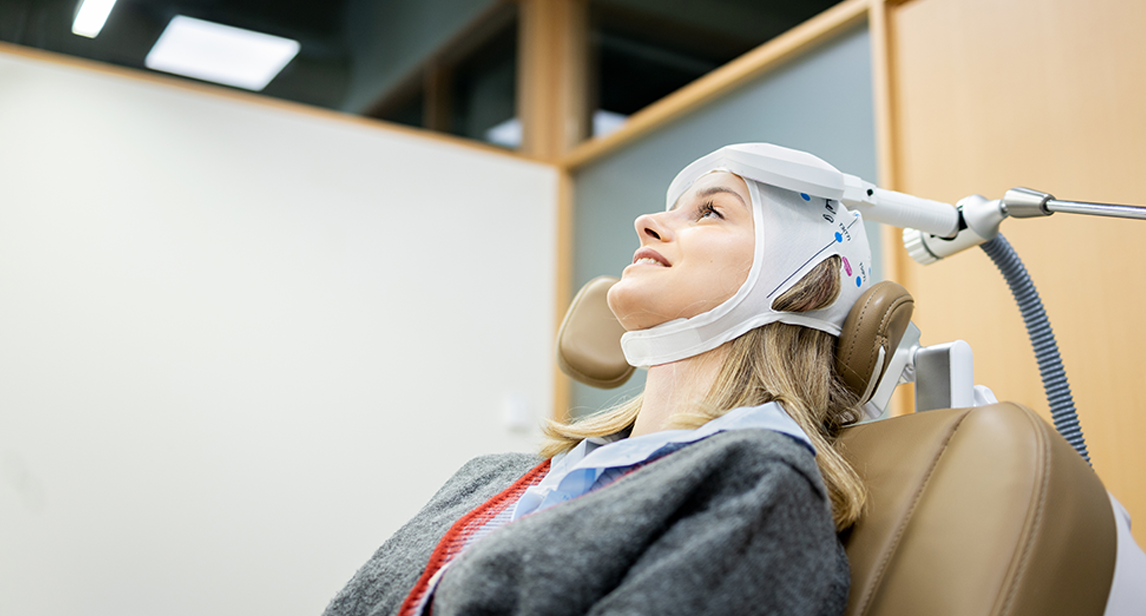
While rTMS is still undergoing ongoing research, clinical trials are being conducted for psychiatric disorders. Clients must meet with study coordinators to review their eligibility criteria to receive treatment. All clients must not have the following to be considered eligible:
Less than one percent of clients may experience a self-limiting seizure. Therefore, clients with a history of seizures (aside from ECT) are not eligible to receive treatment. Some side effects can include:
Neurosync Brain Treatment Centre was the first to introduce MeRT therapy to Ontario. We are a physician-led, fully accredited, medically-based treatment facility providing extensive services and specialized programs to treat a variety of issues like addiction, brain injury, anxiety, depression, and other related matters.
Our treatments are customized to our client’s needs. Our qEEG and EKG scans map each person’s brain, allowing us to focus on exact connectivity areas and functional issues. Treatments vary according to our client’s findings.
We prioritize our client’s experience from their initial consultation through to completion. We want to ensure our clients are fully knowledgeable about the procedure and remain comfortable.
Sed ut perspiciatis unde omnis iste natus error sit voluptatem accusantium doloremque laudantium, totam rem aperiam, eaque ipsa quae ab illo inventore veritatis et quasi architecto beatae vitae dicta sunt explicabo.
Lorem ipsum dolor sit amet,consectr
At vero eos et accusamus et iusto odio dignis
At vero eos et accusamus et iusto odio dignis
To book an appointment to see if you qualify for rTMS therapy, contact us at 416-473-9676 between 9:00 a.m. and 5:00 p.m., Monday through Friday. You can also email us at neurosyncbtc@outlook.com or complete our online form.


Glad I found out about MeRT and even more grateful that it’s in Ontario! Couldn't be more thankful for the team at Neurosync, they took time to explain to me and my husband about Mert therapy and we decided to do the 6 week recommended. I was so amazed that my son who is 6 and non-verbal made such great improvements even beyond the 6 week period!
Anita G


Awesome team and super friendly! Will return for myself next year!
John Doe


The care and attention we received at Neurosync has been outstanding. I highly recommend this place to anyone considering therapy!
Emily R











Please complete the details below and then click on Submit and we’ll be in contact
Working Hours
Email: neurosyncbtc@outlook.com